SOLVED: The product solubility constant for copper (I) iodide is 1.1X 10-12 When 0.40 M HCN is added to this ionic salt; forming the complex ion, Cu(CN) 2 with a product formation constant
Predict which of the ions Cu^+, Sc^3+, Mn^2+, Fe^2+ are coloured in aqueous solution? Give reasons. - Sarthaks eConnect | Largest Online Education Community

Copper Cu transition metal Chemistry copper(I) Cu+ copper(II) Cu2+ ion complex ions ligand substitution compounds redox chemical reactions principal oxidation states +1 +2 GCE AS A2 IB A level inorganic chemistry revision

Highly Selective and Rapid Naked-Eye Colorimetric Sensing and Fluorescent Studies of Cu2+ Ions Derived from Spherical Nanocellulose | ACS Applied Polymer Materials
A copper_ silver cell is set up. The copper ion concentration is 0.10 M. The concentration of silver ion is not know.the cell potential when measured was 0.422V determine the concentration of
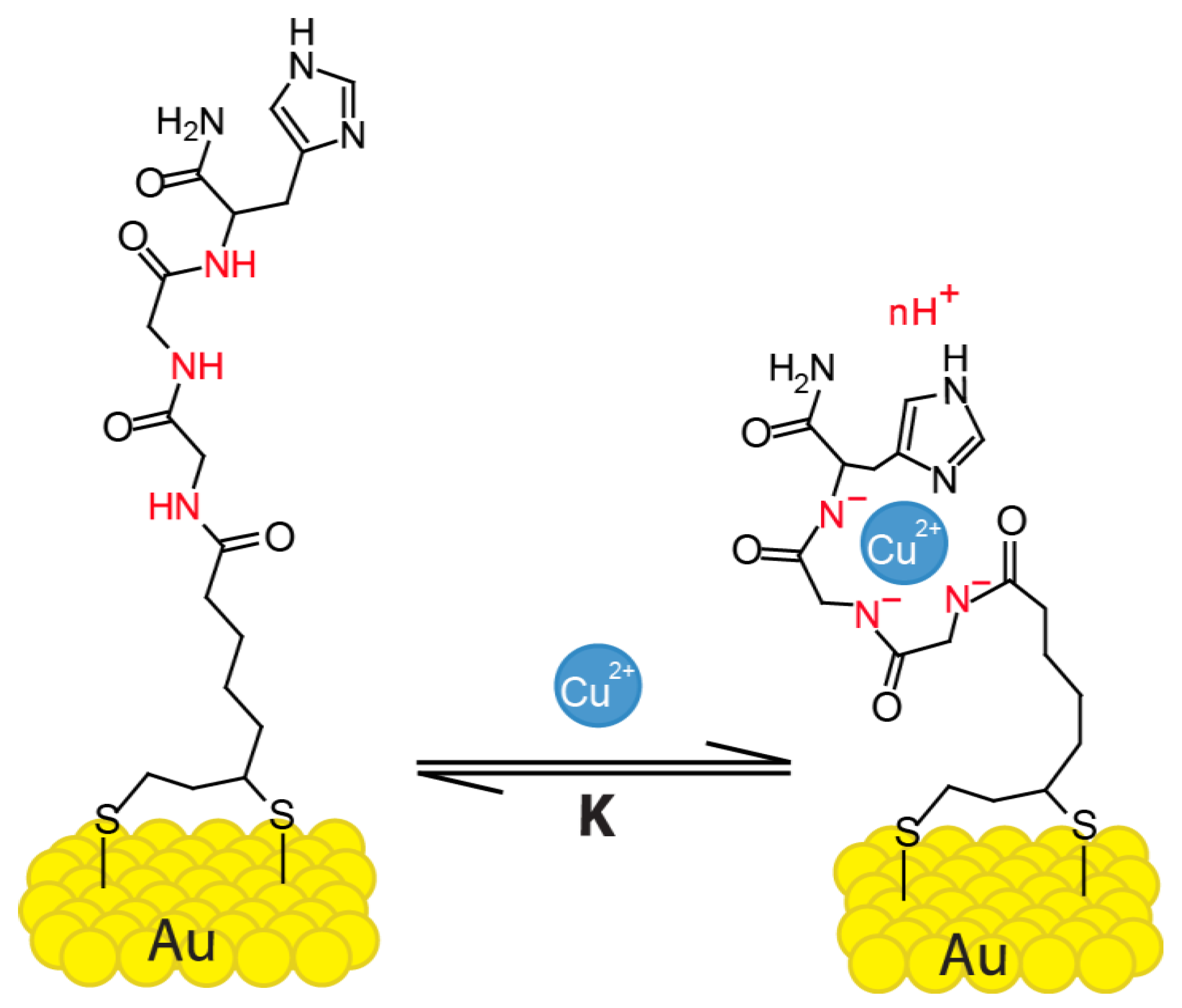
Sensors | Free Full-Text | Detection of Cu2+ Ions with GGH Peptide Realized with Si-Nanoribbon ISFET

Visual Detection of Copper(II) Ions Based on an Anionic Polythiophene Derivative Using Click Chemistry | Analytical Chemistry
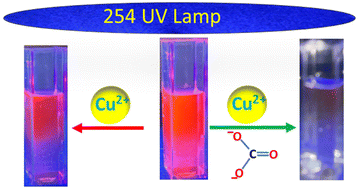
CO32− ion-induced Cu2+ ion determination using DPA capped-LaF3:Eu3+ nanocrystals - Journal of Materials Chemistry C (RSC Publishing)


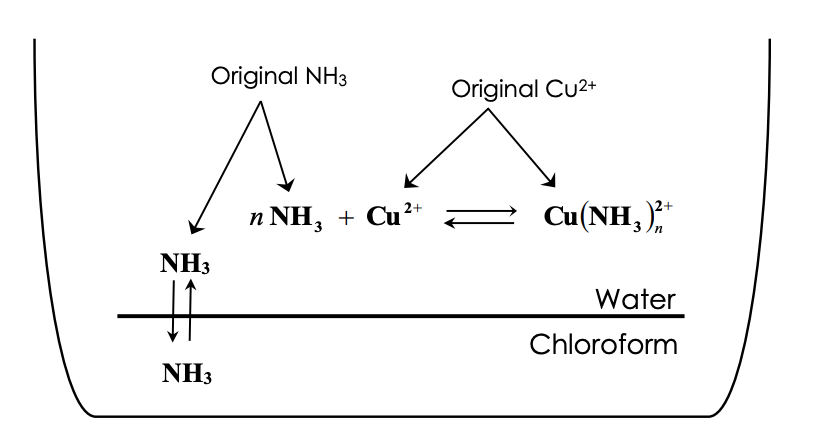
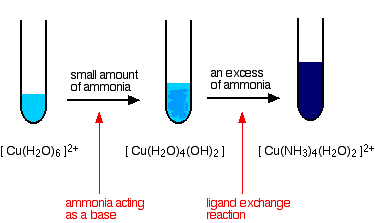
![For a complex ion, [Cu(NH3)4]^2 + : For a complex ion, [Cu(NH3)4]^2 + :](https://haygot.s3.amazonaws.com/questions/1731528_b11046ad9936443990f5efe5a262d1e8.png)